TruSight Oncology IVD Solutions
TruSight Oncology Comprehensive (EU)
Unlock precision medicine with a CE-marked IVD kitted CGP solution, in your lab
Key Benefits:

Generate a Comprehensive Genomic Profile of the Patient Sample
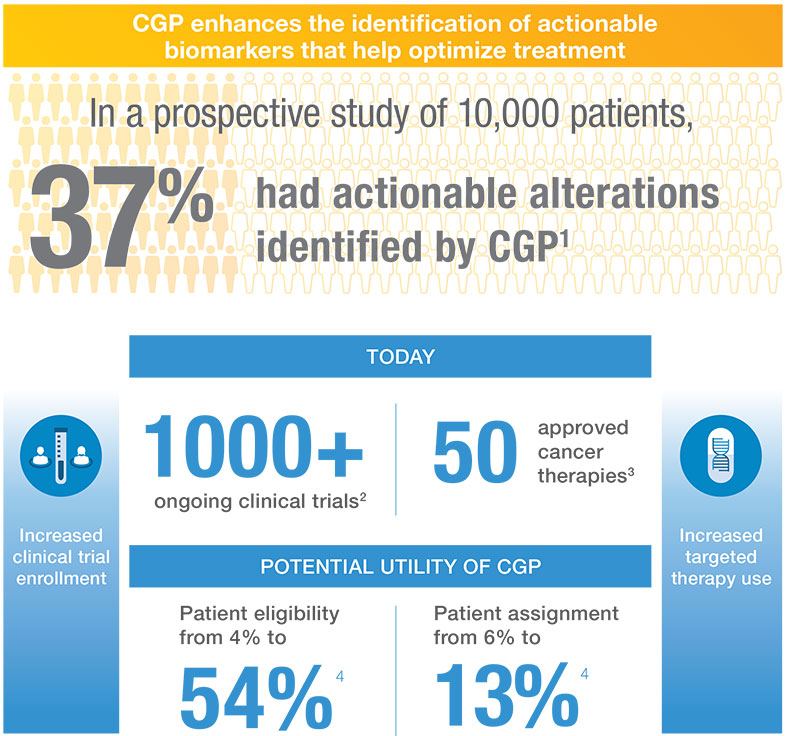
Detect DNA and RNA variants as well as genomic signatures, such as TMB and MSI, for multiple solid tumor types. Generate a comprehensive genomic profile of a patient's tumor to support treatment decisions according to clinical guidelines.

Enable Targeted Therapies Plus Clinical Trials
Content includes key biomarkers associated with drug labels, ESMO recommendations and clinical trials, for multiple solid tumor types. Test results help inform therapy decisions according to clinical guidelines.

Offer Our Test in Your Institution
Offer precision oncology in your institution, keep data in-house and avoid losing samples to send-out services. Internalizing CGP potentially enables your lab to increase the number of informed cases you provide.
Behind Every Test is a Patient
TruSight Oncology Comprehensive (EU) offers cancer patients and healthcare providers the ability to help unlock treatment options and match patients with promising genomically matched therapy regimens. Learn how this IVD test can empower you to be an active voice in making therapy decisions that may improve the patient's journey.
View VideoConserve Biopsy Samples & Consolidate Biomarker Testing
TruSight Oncology Comprehensive (EU) generates a single actionable report with information on 500+ genes in just 4-5 days. Conventional tests often require separate tests and several weeks to obtain reports for a small number of biomarkers.


Maximize the Ability to Detect Actionable Variants
TruSight Oncology Comprehensive (EU) provides coverage of multiple variant classes from DNA and RNA in coding regions of a large panel of cancer-related genes, and genomic signatures like TMB and MSI. Small panels cover fewer genes and less of the coding sequence and may miss actionable alterations.
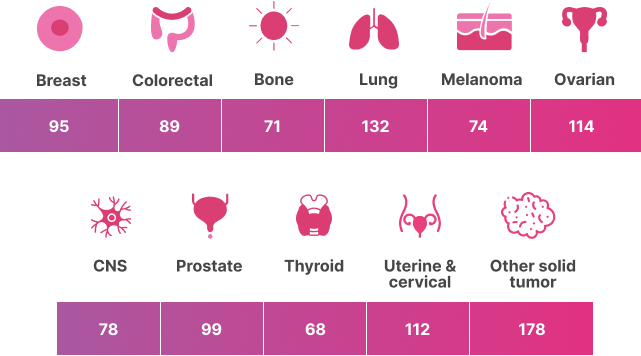
Key Biomarkers
Selection of key biomarkers detected by TruSight Oncology Comprehensive (EU) includes:
Pan-Cancer biomarkers:
BRAF NTRK1 NTRK2 NTRK3 RET MSI TMB
 Breast Breast |
BRCA1 | BRCA2 | ERBB2 | ESR1 | PALB2 | PIK3CA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Colorectal Colorectal |
ERBB2 | KRAS | NRAS | ||||||||
 Bone Bone |
EGFR | ERG | ETV1 | ETV4 | EWSR1 | FEV | FLI1 | FUS | H3F3A | HEY1 | IDH1 |
| MDM2 | NCOA2 | SMARCB1 | |||||||||
 Lung Lung |
ALK | EGFR | ERBB2 | KRAS | MET | NUTM1 | ROS1 | ||||
 Melanoma Melanoma |
KIT | NRAS | ROS1 | ||||||||
 Ovarian Ovarian |
BRCA1 | BRCA2 | FOXL2 | ||||||||
 CNS CNS |
APC | ATRX | CDKN2A | CDKN2B | EGFR | H3F3A | HIST1H3B | HIST1H3C | IDH1 | IDH2 | MYCN |
| PTCH1 | RELA | TERT | TP53 | ||||||||
 Prostate Prostate |
AR | ATM | BARD1 | BRCA1 | BRCA2 | BRIP1 | CDK12 | CHEK1 | CHEK2 | FANCL | FGFR2 |
| FGFR3 | PALB2 | PTEN | RAD51B | RAD51C | RAD51D | RAD54L | |||||
 Thyroid Thyroid |
HRAS | KRAS | NRAS | TERT | |||||||
 Uterine Uterine |
BRCA2 | EPC1 | ERBB2 | ESR1 | FOXO1 | GREB1 | JAZF1 | NCOA2 | NCOA3 | NUTM2A | NUTM2B |
| PAX3 | PAX7 | PHF1 | POLE | SMARCA4 | SUZ12 | TP53 | YWHAE | ||||
 Other Solid Tumors Other Solid Tumors |
ALK | APC | ARID1A | ASPSCR1 | ATF1 | ATIC | BAP1 | BCOR | BRCA1 | BRCA2 | CAMTA1 |
| CARS | CCNB3 | CDK4 | CDKN2A | CIC | CITED2 | CLTC | COL1A1 | COL6A3 | CREB1 | CREB3L1 | |
| CREB3L2 | CSF1 | CTNNB1 | DDIT3 | DDX3X | DNAJB1 | DUX4 | EED | EGFR | ERBB2 | ERG | |
| ETV1 | ETV4 | ETV6 | EWSR1 | FEV | FGFR2 | FGFR3 | FLI1 | FOXL2 | FOXO1 | FOXO4 | |
| FUS | GLI1 | HEY1 | HGF | HMGA2 | IDH1 | KRAS | LEUTX | MAML3 | MDM2 | MYB | |
| MYOD1 | NAB2 | NCOA2 | NF1 | NFATC2 | NFIB | NR4A3 | NRAS | NUTMI | NUTM2A | NUTM2B | |
| PALB2 | PATZ1 | PAX3 | PAX7 | PDGFB | PDGFRA | PRKACA | PRKD1 | RANBP2 | ROS1 | SDHA | |
| SDHB | SDHC | SDHD | SMARCB1 | SS18 | SSX1 | SSX2 | SSX4 | STAT6 | SUZ12 | TAF15 | |
| TCF12 | TERT | TFE3 | TFEB | TFG | TP53 | TPM3 | TPM4 | TRAF7 | TSPAN31 | VGLL2 | |
| WT1 | WWTR1 | YAP1 | YWHAE | ZC3H7B |
Genes listed in this table represent a subset of all genes present in the panel and include genes with biomarkers of clinical significance linked to current drug labels or guidelines. For a full gene list, refer to the product data sheet or package insert. Content analysis provided by Pierian, based on IVD software Knowledge Base v8.5 (February 2023).
From sample to report in just 4 to 5 days
Rely on a CE-marked IVD sample-to-answer solution that can be implemented easily, empowering you to generate test results quickly and accurately.
Fully automated sequencing and data analysis


TruSight Oncology Comprehensive (EU): Request Pricing
See TruSight Oncology Comprehensive (EU) product specifications, available configurations and accessories, and initiate an order online. Take the next step to begin offering this test in your institution.
Request Pricing
Enhance Your Practice - Bring the Test into Your Lab
- Offer a turnaround time of 4-5 days versus two weeks or more for send- out testing.
- Automatically generate an intuitive report - no additional resources needed.
- More cost-effective than send-out testing if your lab runs over 300 samples a year.
- Maintain control of the original samples and data; reanalyze data when a new guideline, drug, or clinical trial becomes available.
- Reduce implementation efforts with validated IVD reagents.
- Improve access by offering tests at locations closer to the patient.
Hear from Our Experts

About Precision Medicine
In this educational video, our healthcare experts discuss the evolution in cancer therapies and how precision medicine, through comprehensive genomic profiling testing, is poised to become the new standard of care.

About TruSight Oncology Comprehensive (EU)
Our physicians, scientists, and product development experts discuss how this new comprehensive genomic profiling test provides extensive content to cover the key biomarkers that can help inform therapy selection decisions for cancer patients.
Comprehensive Onboarding Program
A comprehensive support program is available to expedite implementation and certification and ensure a smooth integration. The program provides laboratory training, including wet-lab instruction and run assessment, a verification protocol, and 24/5 technical support.

Onboarding plan to expedite test verification

Laboratory training, including wet-lab instruction and assessment run

Training Certification

Verification protocol provided

VIP portal for sharing educational and marketing assets by Illumina

Technical support available 24/5
Pipeline of Companion Diagnostic Indications Under Development
TruSight Oncology Comprehensive (EU) is also indicated as a companion diagnostic test to identify cancer patients with solid tumors who are positive for NTRK1, NTRK2, or NTRK3 gene fusions, for treatment with Vitrakvi® (larotrectinib) in accordance with the approved therapeutic labeling.
Content in TruSight Oncology Comprehensive will continue to evolve as new discoveries are made. Illumina has partnered with multiple established pharma companies to develop a growing pipeline of companion diagnostic indications.
Pipeline of Companion Diagnostic Indications Under Development
HER2 RET EGFR ROS-1 HRD HRAS MSI Others
All companion diagnostic tests are currently under development. Availability of each companion diagnostic test is subject to receipt of appropriate regulatory approvals, variable timelines, and geography.
Request a product overview from an oncology specialist for details on the biomarker content, workflow, performance data, and more.
Featured News
Illumina Partners with Bristol Myers Squibb, Kura Oncology, Myriad Genetics, and Merck
Illumina Supports Evaluation of CGP Potential in Late-Stage Cancer Across Belgium
Illumina Partners with Merck on Companion Diagnostic and Research Tests to Identify Cancer Mutations
Illumina and Boehringer Ingelheim Announce Companion Diagnostic Partnership
Related Content

TruSight Oncology Comprehensive (EU) Information Package
Looking for more details on how TruSight Oncology Comprehensive (EU) can benefit your lab? Find detailed specifications and a sample clinical report here.

Key Opinion Leaders Discuss CGP
Healthcare experts worldwide discuss how CGP can lead to more efficiencies in a pathology lab operation, as well as increase the likelihood of finding actionable biomarkers
References
- Content analysis provided by PierianDx Knowledgebase v6.5, September 2021.