
Order the NovaSeq X Series
Advanced chemistry, optics, and informatics combine to deliver exceptional speed and data quality, outstanding throughput and scalability.
FDA-regulated, CE-marked in vitro diagnostic (IVD) next-generation sequencing assay conveniently providing two cystic fibrosis tests in one product.
This product is only available in select locations. Please select the location where you would like this product to be shipped to see availability.
MiSeqDx Reagent Kit v3, Micro provides a flexible, cost-saving option for running a smaller batch size of 24-36 samples. This product is currently validated for exclusive use with the TruSight Cystic Fibrosis 139-Variant Assay. Available in select geographies.
TruSight Cystic Fibrosis is an FDA-regulated in vitro diagnostic (IVD) NGS solution that consolidates two cystic fibrosis assays into a single workflow.
Accurately detects 139 clinically relevant CFTR variants defined in the CFTR2 database as of August 2013.1
Captures all variants in the protein coding regions and intron/exon boundaries for a complete view of the CFTR gene.
| Assay time | 2.5 days |
|---|---|
| Hands-on time | 3.5 hr |
| Input quantity | 250 ng genomic DNA |
| Instruments | MiSeqDx Instrument |
| Method | Targeted DNA Sequencing, Amplicon Sequencing |
| Nucleic acid type | DNA |
| Sample throughput | 24–96 samples per sequencing run |
| Species category | Human |
| Technology | Sequencing |
Optional products:
TruSight Cystic Fibrosis is an FDA-regulated, CE-marked IVD NGS assay that provides two cystic fibrosis tests for carrier screening, confirmatory diagnostic testing of newborns and children, initial testing to aid in diagnosis (TruSight Cystic Fibrosis 139-Variant Assay), and testing in atypical presentations or when other panels have failed to identify causative mutations (TruSight Cystic Fibrosis Clinical Sequencing Assay).
TruSight Cystic Fibrosis 139-Variant Assay
TruSight Cystic Fibrosis Clinical Sequencing Assay
MiSeqDx Reagent Kit v3, Micro
Illumina offers next-generation sequencing-based molecular diagnostic options for clinical laboratories.
FDA-cleared next-generation sequencing panels for detection of causative and clinically relevant variants for cystic fibrosis.
With targeted resequencing, a subset of genes or a genomic region is isolated and sequenced, which can conserve lab resources.
TruSight Cystic Fibrosis 139-Variant Assay
The Illumina TruSight Cystic Fibrosis 139-Variant Assay is a qualitative in vitro diagnostic system used to simultaneously detect 139 clinically relevant cystic fibrosis disease-causing mutations and variants of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in genomic DNA isolated from human peripheral whole blood samples. The variants include those recommended in 2004 by the American College of Medical Genetics (ACMG)4 and in 2011 by the American College of Obstetricians and Gynecologists (ACOG).5
The test is intended for carrier screening in adults of reproductive age, in confirmatory diagnostic testing of newborns and children, and as an initial test to aid in the diagnosis of individuals with suspected cystic fibrosis. The results of this test are intended to be interpreted by a board-certified clinical molecular geneticist or equivalent and should be used in conjunction with other available laboratory and clinical information. This test is not indicated for use for newborn screening, fetal diagnostic testing, pre-implantation testing, or for stand-alone diagnostic purposes.
The test is intended to be used on the Illumina MiSeqDx instrument.
TruSight Cystic Fibrosis Clinical Sequencing Assay
The Illumina TruSight Cystic Fibrosis Clinical Sequencing Assay is a targeted sequencing in vitro diagnostic system that re-sequences the protein coding regions and intron/exon boundaries of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in genomic DNA isolated from human peripheral whole blood specimens collected in K2EDTA. The test detects single nucleotide variants and small indels within the region sequenced, and additionally reports on two deep intronic mutations and two large deletions. The test is intended to be used on the Illumina MiSeqDx instrument.
The test is intended to be used as an aid in the diagnosis of individuals with suspected cystic fibrosis (CF). This assay is most appropriate when the patient has an atypical or non-classic presentation of CF or when other mutation panels have failed to identify both causative mutations. The results of this test are intended to be interpreted by a board-certified clinical molecular geneticist or equivalent and should be used in conjunction with other available information including clinical symptoms, other diagnostic tests, and family history. This test is not indicated for use for stand-alone diagnostic purposes, fetal diagnostic testing, preimplantation testing, carrier screening, newborn screening, or population screening.
For In Vitro Diagnostic Use
Contact an Illumina representative for regional availability.
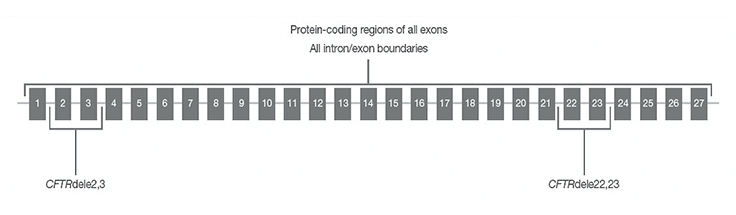

CFTR regions sequenced with TruSight Cystic Fibrosis Clinical Sequencing Assay
CFTR regions sequenced by the assay include protein coding regions across all exons, intron/exon boundaries, ~100 nt of flanking sequence at the 5’ and 3’ UTRs, two deep intronic mutations (1811+1.6kbA>G, 3489+10kbC>T), two large deletions (CFTRdele2,3, CFTRdele22,23) and the PolyTG/PolyT region.
Clinically relevant CFTR variants on TruSight Cystic Fibrosis 139-Variant Assay
TruSight Cystic Fibrosis 139-Variant Assay detects 139 clinically relevant CFTR variants1
| Mutations in the ACMG-23 list for CF screening | ||
| R347P | 1717-1G>A | 3849+10kbC>T |
| G85E | G542X | W1282X |
| R117H | G551D | 711+1G>T |
| 621+1G>T | R553X | R560T |
| R334W | 2184delA | 1898+1G>A |
| A455E | 2789+5G>A | N1303K |
| I507del | 3120+1G>A | R1162X |
| F508del | 3659delC | |
Only a subset of variants included in the assay are listed. To view the full list of variants in the TruSight Cystic Fibrosis 139-Variant Assay, visit www.illumina.com/ TruSightCysticFibrosis.
TruSight Cystic Fibrosis Performance
| TruSight Cystic Fibrosis 139-Variant Assay | |||
| Characteristic | PAa | NAb | OAc |
| Accuracy | 100% | > 99.99% | > 99.99% |
| Reproducibility | 99.77% | 99.88% | 99.88% |
| TruSight Cystic Fibrosis Clinical Sequencing Assay | |||
| Characteristic | PAa | NAb | OAc |
| Accuracy | 99.66% | > 99.99% | > 99.99% |
| Reproducibility | 99.22% | 99.70% | 99.70% |
a. Positive Agreement (PA) is the number of samples with agreeing variant calls divided by the total number of samples with that variant as identified by the reference method.
b. Negative Agreement (NA) calculated across all wild-type (WT) positions by dividing the number of concordant WT positions by the total number of WT positions as defined by the reference methods.
c. Overall Agreement (OA) calculated across all reported positions by dividing the number of concordant wild-type and variant positions by the total number of reported positions as determined by the reference methods.
TruSight Cystic Fibrosis Library Prep
20036925
Supports both the TruSight Cystic Fibrosis 139-Variant Assay and the TruSight Cystic Fibrosis Clinical Sequencing Assay. Includes reagents for library preparation of genomic DNA isolated from 96 peripheral whole blood specimens. Purchase MiSeqDx sequencing reagents separately.
List Price:
Discounts:
MiSeq Dx Reagent Kit v3
20037124
Includes one flow cell and one prefilled cartridge containing clustering and sequencing reagents to support one run of sample libraries on the MiSeqDx System.
List Price:
Discounts:
MiSeq™Dx Reagent Kit v3, Micro
20063860
Includes one micro flow cell and one pre-filled cartridge containing clustering and sequencing reagents to support 24–36 prepared libraries on the MiSeqDx System when using the TruSight Cystic Fibrosis Library Prep.
List Price:
Discounts:
TruSeq Index Plate Fixture Kit (2 fixtures)
FC-130-1005
Assists in correctly arranging index primers for PCR amplification step. Includes two (2) TruSeq Index Plate Fixtures.
List Price:
Discounts:
TruSeq Index Plate Fixture & Collar Kit (2 each)
FC-130-1007
Assists in correctly arranging index primers for PCR amplification step. Includes two (2) TruSeq Index Plate Fixtures and two (2) Plate Collars.
List Price:
Discounts:
Showing of
Product
Qty
Unit Price
Product
Catalog ID
Quantity
Unit price
TruSight Cystic Fibrosis 139-Variant Assay detects 139 variants in the CFTR gene for carrier screening, confirmatory diagnostic testing of newborns and children, and initial diagnostic testing.
TruSight Cystic Fibrosis Clinical Sequencing Assay re-sequences the protein coding region and intron/exon boundaries of the CFTR gene for aiding in diagnosis, testing in atypical presentations and when other panels do not identify both causative mutations.
Yes, prepared libraries stored as diluted amplicon libraries (DALs) can be used up to 28 days after preparation when stored frozen. There is no difference between libraries prepared for use in the TruSight Cystic Fibrosis 139-Variant Assay and TruSight Cystic Fibrosis Clinical Sequencing Assay.
Yes, you can do reflex testing from the TruSight Cystic Fibrosis 139-Variant Assay to the TruSight Cystic Fibrosis Clinical Sequencing Assay when the patient has an atypical or nonclassical presentation of CF or when other mutation panels have failed to identify both causative mutations.
Both TruSight Cystic Fibrosis assays have been designed to run with the MiSeqDx Reagent Kit v3. The MiSeqDx v3 sequencing flow cell has been validated to support 24-96 tests of each assay per sequencing run. The MiSeqDx Reagent Kit v3, Micro supports batches of 24-36 samples. TruSight Cystic Fibrosis has been validated to support a minimum of 24 samples per flow cell run.
References:
1. Clinical and Functional Translation of CFTR. www.cftr2.org. Accessed August 2013.
2. Sosnay PR, Siklosi KR, Van Goor F, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nature Genetics. 2013;45(10):1160-1167. doi:10.1038/ng.2745
3. Hughes EE, Stevens CF, Saavedra-Matiz CA, et al. Clinical sensitivity of cystic fibrosis mutation panels in a diverse population. Human Mutation. 2016;37(2):201-208. doi:10.1002/humu.22927
4. Watson MS, Cutting GR, Desnick RJ, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med. 2004;6(5):387-391. doi:10.1097/01.gim.0000139506.11694.7c
5. Committee on Genetics. The American College of Obstetricians and Gynecologists Committee Opinion No. 486: Update on carrier screening for cystic fibrosis. Obstet Gyncol. 2011;486:1-4. doi:https://pubmed.ncbi.nlm.nih.gov/21422883/
Reach out for information about our products and services, or get answers to questions about our technology.
Your email address is never shared with third parties.