NIPT Sendout Labsのリソース
出生前スクリーニングの最前線
ラボで非侵襲的出生前検査(NIPT)を提供することに関心をお持ちの場合は、ラボソリューションとラボ外ソリューションの2つの製品を提供しています。NIPTソリューションをラボ内に導入したいラボ向けに、VeriSeq NIPT Solutionが、いくつかの欧州連合加盟国で利用可能になりました。製品ポートフォリオの一部としてNIPTを提供し、処理についてはイルミナCLIAラボにサンプルを送ることをご希望される場合、Verifi Prenatal Testは優れた選択肢です。

プロセスの仕組み
当社のVerifi NIPTサービスは、サンプルから回答までシームレスなプロセスを実現します。

検査依頼書/署名入りの同意書および血液サンプルが取得されます。

血液サンプルおよび検査依頼書は、ラボに返送されます。

ラボは、サンプルを処理および分析します。

ラボは、検査結果を報告します。
よくある質問
2017年の初めに、CMSは、NIPTに関連する3つのCPTコードの2017年の価格設定を確定しました。
| CPTコード | 簡単な説明 | 最終CY2017 |
|---|---|---|
| 0009M | 母親の血漿を用いた選択領域の胎児異数性(21トリソミーおよび18トリソミー)DNAシーケンス解析、各トリソミーのリスクスコアとして報告されるアルゴリズム | 602.10ドル |
| 81420 | 胎児の染色体異数性(例:21トリソミー、Xモノソミー)ゲノムシーケンス解析パネル、母体血中循環セルフリー胎児DNAには、13番、18番、21番染色体の解析を含む必要があります | 802.33ドル |
| 81422 | 胎児染色体微小欠失ゲノムシーケンス解析(例:ディジョージ症候群、クリ・デュ・チャット症候群)、母体血液循環セルフリー胎児DNA | 802.33ドル |
胎児異数性リスク*が高い患者は、米国ではほとんどの民間保険および公的保険で補償されます。臨床ガイドラインは継続的に新たなテクノロジーに追従するため、Anthem Blue Cross Blue Shield社およびCigna社などの一部の保険会社では、すべての妊婦に適用範囲を拡大し、NIPT費用を償還する最大の医療保険プランとなっています。
American College of Medical Genetics(ACMG)は、妊娠週数9~10週から連続した妊娠週数において、NIPTが従来の方法でスクリーニングされた13番、18番、21番の異数性に対して最も感度の高いスクリーニングの選択肢であることをすべての妊婦に通知することを推奨しています。米国産科婦人科医会(ACOG)および母体胎児医学会(SMFM)も、以下を推奨しています。「母親の年齢に関係なく、すべての女性に、異数性スクリーニングまたは胎児遺伝性疾患の診断検査の選択肢が与えられるべきです。スクリーニング検査の選択は、分娩前に情報を得たいという希望、以前の産科既往歴、家族歴、胎児の数など、多くの要因の影響を受けます。あらゆる検査特性において、どの検査が優れているというわけではなく、加えて、すべてのセンターですべての検査が受けられるわけでもありません。」
*ACOGおよびSMFMのガイドライン1によると、高リスクは、以下の要因に関連しています。
- 母親の年齢が35歳以上
- 親の染色体転座
- 以前の子どもがトリソミーを持っている
- 超音波検査における重大な所見
- スクリーニング検査陽性
診療所の所在地や医療保険制度へのアクセス状況に応じて、患者がNIPTの補償対象かどうかについては、保険プラン管理者にご相談ください。
1.Practice Bulletin 163 Screening for Fetal Aneuploidy (May 2016)
NIPTの償還率は、保守契約により各保険プランで異なります。NIPTの定価は、1回の検査につき、1,000ドル未満から2,000ドル超まで幅があります。調査によると、NIPTを第一選択のスクリーニングとして利用する場合、619~744ドル1-3の費用対効果があり、この価値の大部分は、21トリソミー3のスクリーニングによるものです。NIPTは、一般的な妊娠(周産期)母集団で利用する場合、コストを節約できます。
1.Benn P, Curnow KJ, Chapman S, Michalopoulos SN, Hornberger J, Rabinowitz M. An Economic Analysis of Cell-Free DNA Non-Invasive Prenatal Testing in the US General Pregnancy Population. PLoS One. 2015;10(7):e0132313.
2.Fairbrother G, Burigo J, Sharon T, Song K. Prenatal screening for fetal aneuploidies with cell-free DNA in the general pregnancy population: a cost-effectiveness analysis. J Matern Fetal Neonatal Med.(母子医学ジャーナル。) 2016;29(7):1160-1164.
3.Walker BS, Nelson RE, Jackson BR, Grenache DG, Ashwood ER, Schmidt RL. A Cost-Effectiveness Analysis of First Trimester Non-Invasive Prenatal Screening for Fetal Trisomies in the United States. PLoS One. 2015;10(7):e0131402.
NIPTの開発が始まって以来、臨床および技術の専門家や医学会によって、60以上の出版物が作成、発表されています。あなたやあなたの同僚がより簡単に決断できるよう、当社は現在利用可能な研究、ガイドライン、参考文献を臨床関連書類に要約しておりますので、ダウンロードして確認いただけます。
NIPTの償還に関する詳細情報は、NIPTreimbursement@illumina.comまでEメールでお問い合わせください。

Additional Resources

Implications of Test Failures: A Webinar with Professor Yuval Yaron
Professor Yuval Yaron discusses the implications of test failures.
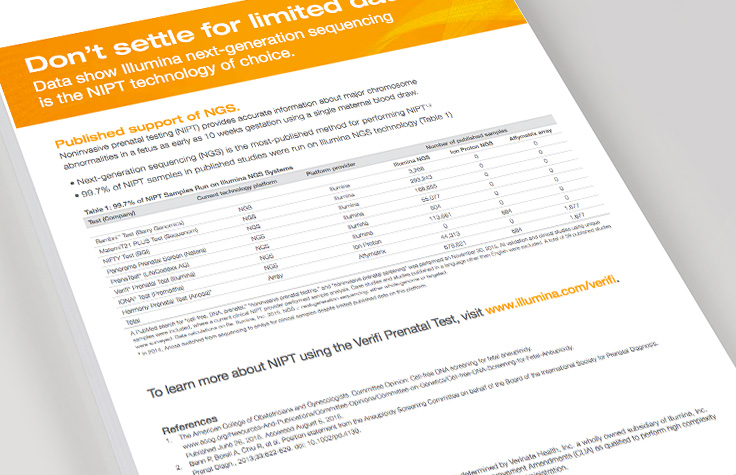
Don't Settle for Limited Data
Data shows Illumina next-generation sequencing is the NIPT technology of choice.
参考文献
- Practice Bulletin No. 163: Screening for Fetal Aneuploidy. Obstet Gynecol. 2016;127(5):979-981.
Verifi Prenatal Testは、イルミナの完全子会社であるVerinata Health, Inc.によって開発され、性能特性が決定されました。VHIラボは、米国臨床病理医協会認定を受けており、Clinical Laboratory Improvement Amendments(CLIA)認可の下、複雑度の高い臨床検査を行っています。これはアメリカ食品医薬品局の認可または承認を受けていません。