クロマチンアクセシビリティ解析用のATAC-Seq
ATAC-Seq (Assay for Transposase-Accessible Chromatin with Sequencing)
ハイスループット機能を備え、ゲノム全体のアクセス可能なクロマチンを迅速かつ高感度にプロファイリングする手法
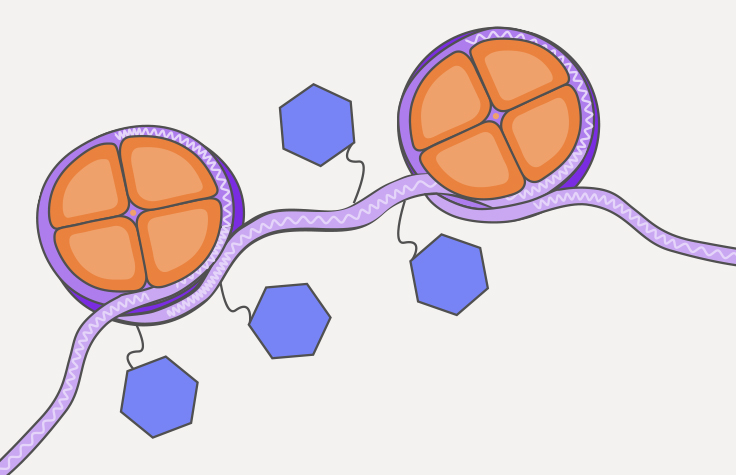
ATAC-Seqとは?
ATAC-Seq(Assay for transposase-accessible chromatin with sequencing)は、ゲノム全体のクロマチンアクセシビリティを決定するための一般的な方法です。オープンクロマチン領域をシーケンスすることで、ATAC-Seqはクロマチンのパッケージングやその他の要因が遺伝子発現にどのように影響するかを明らかにするのに役立ちます。
ATAC-Seqは、規制要素に関する事前知識を必要としないため、強力でエピジェネティックな探索ツールとなります。複雑な疾患、胚発生、T細胞活性化、がんにおけるクロマチンアクセシビリティ、転写因子結合、遺伝子制御の理解を深めるために用いられています1,2。ATAC-Seqは、バルク細胞集団やシングルセルに対して高解像度で実施できます。
ATAC-Seqの仕組みは?
ATAC-Seqでは、ゲノムDNAは高活性トランスポザーゼであるTn5に曝されます。Tn5は同時にDNAを断片化し、オープンクロマチン部位に優先的に挿入し、シーケンスプライマーを付加(タグメンテーションと呼ばれるプロセス)します。DNAのシーケンスによってオープンクロマチンを同定でき、データ解析によって遺伝子制御に対する洞察が得られます。
さらに、ATAC-Seqは、遺伝子発現を研究するためのマルチオミクスアプローチとして、RNAシーケンスなどの他の手法と組み合わせることができます3,4。後続の実験には、エピジェネティックな制御の形態をさらに特徴づけるために、ChIP-Seq、Methyl-Seq、Hi-C-Seqなどが用いられることがよくあります。
シングルセル向けATAC-Seqの適用方法
スタンフォード大学のDr. William Greenleafが、ATAC-Seqの開発とシングルセルへの適用について述べます。さらに、ゲノミクスとエピジェネティクスの専門家であるDr. Bing RenとDr. Sebastian Preisslが、コンビナトリアルインデックスを用いたシングルセルATAC-Seqについて述べます。
ウェビナーを視聴する
ATAC-Seqの主要なアプリケーション
ATAC-Seqによるクロマチンアクセシビリティ解析は、ゲノムの制御環境に対する貴重な知見を提供します。定評のあるアプリケーションは以下のとおりです。
- ヌクレオソームマッピング
- 転写因子結合解析
- 新規エンハンサーの同定
- 疾患関連の制御メカニズムの探索
- 細胞型特異的制御解析
- 進化学研究
- 比較エピゲノミクス
- バイオマーカーの発見

NGSが遺伝子制御とトランスクリプトミクス研究を加速する
NGSがどのように遺伝子発現と制御の研究をさまざまな分野で進めているかを解説します。ATAC-Seqやメチル化シーケンスなどの手法を用いた遺伝子制御研究が、RNAシーケンスをどのように補完できるかをご覧ください。
eBookをダウンロードATAC-Seqの推奨カバレッジ
ATAC-Seqに必要な最低限のシーケンスカバレッジは研究目的によって異なります。この表には、一般的なアプリケーションに関するガイドラインが記載されています。
ATAC-Seqにはペアエンドシーケンスリードの使用を推奨します。シングルリードシーケンスと比較して、ペアエンドリードには次の利点があります。
- 高いユニークアライメント率
- PCRデュプリケートの除去を改善
- アクセス可能なシーケンスに関する情報がより充実
- リードをヌクレオソームフリー、モノヌクレオソーム、またはジヌクレオソームとして分類する精度が向上
| 研究目標 | 推奨される深度 |
|---|---|
| ヒトサンプルにおけるオープンクロマチンの違いの同定 | 5000万以上のペアエンドリード |
| 遺伝子制御ネットワーク構築のための転写因子フットプリント解析 | 2億を超えるペアエンドリード |
| シングルセル解析 | 核/細胞あたり25,000~50,000のペアエンドリード |
実験のニーズは多様であるため、科学文献を参照して、プロジェクトに適したカバレッジレベルを決定することをお勧めします。
ATAC-Seqを用いた論文
ヒトT細胞における遺伝子制御の解読
サイエンティストがATAC-Seqを用いてクロマチン状態に影響を与える制御因子を同定して遺伝子発現を制御する方法をご覧ください。
ATAC-Seqを使用したメタゲノムアセンブリの改善
研究者がATAC-Seqの実現可能性と利点を実証し、細菌ゲノムの研究を支援する方法をご紹介します。
がんにおけるゲノムアーキテクチャーの特性評価
サイエンティストがATAC-Seqを活用し、The Cancer Genome Atlasの15の原発性ヒトがんタイプを用いてがんのゲノムトポロジーの理解を深める方法をご覧ください。
ATAC-Seqでは、複雑または希少な組織におけるエピジェネティックなばらつきや、これまでゲノムワイドレベルでは観察できなかった細胞集団におけるエピゲノムランドスケープを調べることができます。
補足資料
トランスクリプトームとエピゲノムのプロファイリング
このテクニカルノートでは、10x Genomics Chromium Single Cell Multiome ATAC + Gene Expressionを使用したシングルセルからのトランスクリプトームとエピゲノムの同時プロファイリングについて説明しています。
シロイヌナズナ(A. thaliana)の遺伝子制御ランドスケープ
シロイヌナズナ(Arabidopsis thaliana)のシングルセルATAC-Seqに関するウェビナーにご参加ください。講演者は、植物の発生を制御する遺伝子ネットワークを推定するための分析フレームワークについて解説します。
シングルセルアプリケーションに向けたサンプル調製の最適化
このウェビナーでは、業界の専門家が、ATAC-Seqの他、scRNA-SeqやsNuc-Seqなどのアッセイのサンプル調製技術とベストプラクティスについての知見を明らかにします。
がんエピジェネティクス
ATAC-Seqを使用して、がんのエピジェネティックな特徴を探索する方法と、開始に役立つガイド、製品、その他のリソースをご覧ください。
RNAシーケンス
RNA-Seqは、NGSを使用してトランスクリプトーム全体の発現を解析し、既知または新規の特徴を検出するほか、RNAを定量化することを可能にします。
ATAC-Seqに関するFAQ
参考文献
- Cusanovich DA, Reddington JP, Garfield DA, et al. The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature. 2018; 555:538-542. 13.
- Gate RE, Cheng CS, Aiden AP, et al. Genetic determinants of co-accessible chromatin regions in activated T-cells across humans. Nat Genet. 2018; 50:1140-1150.
- Luo L, Gribskov M, Wang S. Bibliometric review of ATAC-Seq and its application in gene expression. Briefings Bioinform. 2025;23:1-13. doi: 10.1093/bib/bbac061.
- Cao J, Cusanovich DA, Ramani V, et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science. 2018;361:1380-1385.