詳細
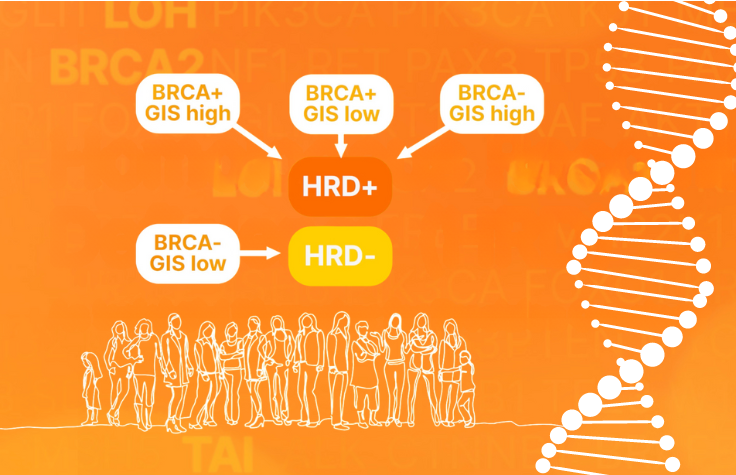
Homologous recombination repair deficiency (HRD) is associated with response to poly-ADP-ribose polymerase inhibition (PARPi) therapy in advanced ovarian cancer. In combination with CGP, these analyses allow clinicians to maximize clinical information so that patients are matched with approved therapies or enrolled in relevant clinical trials.
In this workshop, a pathologist and laboratory director will discuss the impact of implementing a HRD assay at their institution. They will describe the details of implementing the assay, technical considerations for HRD testing, concordance with other HRD assays, and the added value of offering CGP and HRD testing in-house.

Carlo Bifulco, MD
Director of Molecular Pathology and Pathology Informatics
Providence St. Joseph Health

Brian Piening, PhD
Associate Member at Earle A. Chiles Research Institute
Technical Director Clinical Genomics at Providence St. Joseph Health
Fill Out Form to Access Webinar
ご提供いただいた個人情報は、お客様へのサポート、サービス、および販売活動の目的にのみ使用させていただきます。
- 日時
- 2023/11/15
- Location
- Salt Lake City, Utah
- Topic
- Oncology