
NovaSeq Xシリーズのご購入
進化したケミストリー、光学、インフォマティクスを融合させ、卓越したシーケンシング速度とデータ品質、優れたスループットとスケーラビリティをお届けします。
FDA-regulated, CE-marked in vitro diagnostic (IVD) next-generation sequencing assay conveniently providing two cystic fibrosis tests in one product.
この製品は出荷できる国が制限されています。 出荷の可否を確認するには、 出荷する国を選択してください 。
MiSeqDx Reagent Kit v3, Micro provides a flexible, cost-saving option for running a smaller batch size of 24-36 samples. This product is currently validated for exclusive use with the TruSight Cystic Fibrosis 139-Variant Assay. Available in select geographies.
TruSight Cystic Fibrosis is an FDA-regulated in vitro diagnostic (IVD) NGS solution that consolidates two cystic fibrosis assays into a single workflow.
Accurately detects 139 clinically relevant CFTR variants defined in the CFTR2 database as of August 2013.1
Captures all variants in the protein coding regions and intron/exon boundaries for a complete view of the CFTR gene.
| アッセイ時間 | 2.5日 |
|---|---|
| ハンズオンタイム | 3.5時間 |
| インプット量 | 250 ng ゲノムDNA |
| システム | MiSeqDx Instrument |
| 手法 | ターゲットDNAシーケンス, アンプリコンシーケンス |
| 核酸の種類 | DNA |
| サンプルスループット | シーケンスランあたり24~96サンプル |
| 生物種カテゴリー | ヒト |
| テクノロジー | シーケンス |
Optional products:
TruSight Cystic Fibrosis is an FDA-regulated, CE-marked IVD NGS assay that provides two cystic fibrosis tests for carrier screening, confirmatory diagnostic testing of newborns and children, initial testing to aid in diagnosis (TruSight Cystic Fibrosis 139-Variant Assay), and testing in atypical presentations or when other panels have failed to identify causative mutations (TruSight Cystic Fibrosis Clinical Sequencing Assay).
TruSight Cystic Fibrosis 139-Variant Assay
TruSight Cystic Fibrosis Clinical Sequencing Assay
MiSeqDx Reagent Kit v3, Micro
TruSight Cystic Fibrosis 139-Variant Assay
The Illumina TruSight Cystic Fibrosis 139-Variant Assay is a qualitative in vitro diagnostic system used to simultaneously detect 139 clinically relevant cystic fibrosis disease-causing mutations and variants of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in genomic DNA isolated from human peripheral whole blood samples. The variants include those recommended in 2004 by the American College of Medical Genetics (ACMG)4 and in 2011 by the American College of Obstetricians and Gynecologists (ACOG).5
The test is intended for carrier screening in adults of reproductive age, in confirmatory diagnostic testing of newborns and children, and as an initial test to aid in the diagnosis of individuals with suspected cystic fibrosis. The results of this test are intended to be interpreted by a board-certified clinical molecular geneticist or equivalent and should be used in conjunction with other available laboratory and clinical information. This test is not indicated for use for newborn screening, fetal diagnostic testing, pre-implantation testing, or for stand-alone diagnostic purposes.
The test is intended to be used on the Illumina MiSeqDx instrument.
TruSight Cystic Fibrosis Clinical Sequencing Assay
The Illumina TruSight Cystic Fibrosis Clinical Sequencing Assay is a targeted sequencing in vitro diagnostic system that re-sequences the protein coding regions and intron/exon boundaries of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in genomic DNA isolated from human peripheral whole blood specimens collected in K2EDTA. The test detects single nucleotide variants and small indels within the region sequenced, and additionally reports on two deep intronic mutations and two large deletions. The test is intended to be used on the Illumina MiSeqDx instrument.
The test is intended to be used as an aid in the diagnosis of individuals with suspected cystic fibrosis (CF). This assay is most appropriate when the patient has an atypical or non-classic presentation of CF or when other mutation panels have failed to identify both causative mutations. The results of this test are intended to be interpreted by a board-certified clinical molecular geneticist or equivalent and should be used in conjunction with other available information including clinical symptoms, other diagnostic tests, and family history. This test is not indicated for use for stand-alone diagnostic purposes, fetal diagnostic testing, preimplantation testing, carrier screening, newborn screening, or population screening.
For In Vitro Diagnostic Use
Contact an Illumina representative for regional availability.
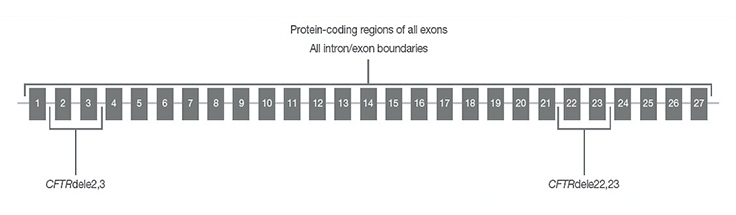

CFTR regions sequenced with TruSight Cystic Fibrosis Clinical Sequencing Assay
CFTR regions sequenced by the assay include protein coding regions across all exons, intron/exon boundaries, ~100 nt of flanking sequence at the 5’ and 3’ UTRs, two deep intronic mutations (1811+1.6kbA>G, 3489+10kbC>T), two large deletions (CFTRdele2,3, CFTRdele22,23) and the PolyTG/PolyT region.
Clinically relevant CFTR variants on TruSight Cystic Fibrosis 139-Variant Assay
TruSight Cystic Fibrosis 139-Variant Assay detects 139 clinically relevant CFTR variants1
| Mutations in the ACMG-23 list for CF screening | ||
| R347P | 1717-1G>A | 3849+10kbC>T |
| G85E | G542X | W1282X |
| R117H | G551D | 711+1G>T |
| 621+1G>T | R553X | R560T |
| R334W | 2184delA | 1898+1G>A |
| A455E | 2789+5G>A | N1303K |
| I507del | 3120+1G>A | R1162X |
| F508del | 3659delC | |
Only a subset of variants included in the assay are listed. To view the full list of variants in the TruSight Cystic Fibrosis 139-Variant Assay, visit www.illumina.com/ TruSightCysticFibrosis.
TruSight Cystic Fibrosis Performance
| TruSight Cystic Fibrosis 139-Variant Assay | |||
| Characteristic | PAa | NAb | OAc |
| Accuracy | 100% | > 99.99% | > 99.99% |
| Reproducibility | 99.77% | 99.88% | 99.88% |
| TruSight Cystic Fibrosis Clinical Sequencing Assay | |||
| Characteristic | PAa | NAb | OAc |
| Accuracy | 99.66% | > 99.99% | > 99.99% |
| Reproducibility | 99.22% | 99.70% | 99.70% |
a. Positive Agreement (PA) is the number of samples with agreeing variant calls divided by the total number of samples with that variant as identified by the reference method.
b. Negative Agreement (NA) calculated across all wild-type (WT) positions by dividing the number of concordant WT positions by the total number of WT positions as defined by the reference methods.
c. Overall Agreement (OA) calculated across all reported positions by dividing the number of concordant wild-type and variant positions by the total number of reported positions as determined by the reference methods.
TruSight Cystic Fibrosis Library Prep
20036925
TruSight Cystic Fibrosis 139-Variant Assayと、TruSight Cystic Fibrosis Clinical Sequencing Assayの両方をサポートします。末梢血全血96サンプルから分離されたゲノムDNAのライブラリー調製用試薬が含まれます。MiSeqDxシーケンス試薬は別売りです。
List Price:
Discounts:
MiSeqDx 試薬キット(MiSeq Dx Reagent Kit v3)
20037124
MiSeqDxシステム上でのサンプルライブラリーラン1回をサポートするためのフローセル1個と、クラスター試薬およびシーケンス試薬を含むプレフィルドカートリッジ1個が含まれます。
List Price:
Discounts:
MiSeq™Dx Reagent Kit v3, Micro
20063860
TruSight Cystic Fibrosis Library Prep を使用する際、MiSeqDxシステムで調製された24~36のライブラリーをサポートするマイクロフローセル1個と、クラスター試薬およびシーケンス試薬を含むプレフィルドカートリッジ1個が含まれます。
List Price:
Discounts:
TruSeq Index Plate Fixture Kit (2 fixtures)
FC-130-1005
PCR増幅ステップでのインデックスプライマーの正確な配置をサポートします。TruSeq Index Plate Fixtures(2個)が含まれます。
List Price:
Discounts:
TruSeq Index Plate Fixture & Collar Kit (2 each)
FC-130-1007
PCR増幅ステップ用のインデックスプライマーの正しい配置を支援します。TruSeqインデックスプレートフィクスチャー(2個)とプレートカラー(2個)が含まれます。
List Price:
Discounts:
表示されている結果 : /
製品名
数量
単価
製品名
カタログ番号
量
単価
TruSight Cystic Fibrosis 139-Variant Assay detects 139 variants in the CFTR gene for carrier screening, confirmatory diagnostic testing of newborns and children, and initial diagnostic testing.
TruSight Cystic Fibrosis Clinical Sequencing Assay re-sequences the protein coding region and intron/exon boundaries of the CFTR gene for aiding in diagnosis, testing in atypical presentations and when other panels do not identify both causative mutations.
Yes, prepared libraries stored as diluted amplicon libraries (DALs) can be used up to 28 days after preparation when stored frozen. There is no difference between libraries prepared for use in the TruSight Cystic Fibrosis 139-Variant Assay and TruSight Cystic Fibrosis Clinical Sequencing Assay.
Yes, you can do reflex testing from the TruSight Cystic Fibrosis 139-Variant Assay to the TruSight Cystic Fibrosis Clinical Sequencing Assay when the patient has an atypical or nonclassical presentation of CF or when other mutation panels have failed to identify both causative mutations.
Both TruSight Cystic Fibrosis assays have been designed to run with the MiSeqDx Reagent Kit v3. The MiSeqDx v3 sequencing flow cell has been validated to support 24-96 tests of each assay per sequencing run. The MiSeqDx Reagent Kit v3, Micro supports batches of 24-36 samples. TruSight Cystic Fibrosis has been validated to support a minimum of 24 samples per flow cell run.
References:
1. Clinical and Functional Translation of CFTR. www.cftr2.org. Accessed August 2013.
2. Sosnay PR, Siklosi KR, Van Goor F, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nature Genetics. 2013;45(10):1160-1167. doi:10.1038/ng.2745
3. Hughes EE, Stevens CF, Saavedra-Matiz CA, et al. Clinical sensitivity of cystic fibrosis mutation panels in a diverse population. Human Mutation. 2016;37(2):201-208. doi:10.1002/humu.22927
4. Watson MS, Cutting GR, Desnick RJ, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med. 2004;6(5):387-391. doi:10.1097/01.gim.0000139506.11694.7c
5. Committee on Genetics. The American College of Obstetricians and Gynecologists Committee Opinion No. 486: Update on carrier screening for cystic fibrosis. Obstet Gyncol. 2011;486:1-4. doi:https://pubmed.ncbi.nlm.nih.gov/21422883/
Reach out for information about our products and services, or get answers to questions about our technology.
ご提供いただいた個人情報は、お客様へのサポート、サービス、および販売活動の目的にのみ使用させていただきます。